In his last talk at Bio-IT World, Dr. Terry Barnhart spoke of the potential of Agile practices in pharmaceutical innovation. At the most recent Bio-IT World conference, Barnhart shows how Agile practices not only bring products to market faster – they also save lives. As we have discussed in previous Lean Coffees about the intersections between Agile practices and Agile Biopharma, organizations can incorporate Agile practices without using the “Agile” label or terminology, and a major example is Operation Warp Speed, which helped bring vaccines to market quickly to help fight the spread of COVID-19.
Barnhart began his talk by reflecting on his 20 years of work in the pharma industry, most recently in his organizational role at Novartis and within R&D at Pfizer. He noted that the industry has made considerable gains to save lives and reduce suffering, as evidenced by the 19,000 prescription medications and 340 biologics available in the United States.
Agile Biopharma – Feeling Stuck
While the pharmaceutical industry spends billions on R&D, generates about $118 billion in profit, and has a global impact, Barnhart wondered why the industry isn’t innovating even more: “Why do we feel stuck? Just about everyone that I talk to in the industry feels this, like we’re stuck in molasses, like our feet are just chained to the floor?” He pointed out that the median years to market for a new medication is nine years – and that hasn’t changed for 20 years.
A possible reason for feeling stuck is that companies are growing larger, which means scientists are working on six or seven programs at a time, and in at least one case, it takes as many as 50 approvals to get to a stage gate. As Barnhart put it, “That sort of stuff is not the stuff of which speed is made.” Even more difficult, it means there are many players that scientists have to engage with before they can deliver a medicine to a patient.
The pharma industry is always running programs to accelerate R&D, but that hasn’t moved the needle on the nine years it takes to get a new medication to market. Meanwhile, program leaders are begging for resources, and when change leaders like Barnhart try to make a difference, they often encounter the following responses to their ideas, such as A) “It’ll never help” or B) “It’s getting in the way of speed to the next place.” Finally, Barnhart said, “Employees at all levels will tell you that it’s just like running through muck.”
Where Operation Warp Speed Fits In
The pressure of COVID-19 and the 30,000 lives lost every month forced companies to change their processes out of necessity. Barnhart noted that six different companies worked on the vaccine and walked through the incredible timeline from mapping the genome to releasing the vaccine:
- January 11-12: COVID-19 genome is available
- January 15: Companies start work on the vaccines
- October: 2-month safety data is complete
- November: Submissions made to the FDA
- December: Emergency authorizations made for Pfizer and Moderna
What was typically a 5- to 17-year process for vaccine authorization had been compressed into 11 months, proving that it is possible to bring medicines to market faster.
How did this get done? Barnhart said companies “looked at every barrier on the way from idea to launch and they got rid of it. They looked at everything that could go in parallel and they made it go in parallel. They looked at every approval that was on the way. And they made sure that there was no waiting.”
“[Companies] looked at every barrier on the way from idea to launch and they got rid of it. They looked at everything that could go in parallel and they made it go in parallel. They looked at every approval that was on the way, and they made sure that there was no waiting.” — Dr. Terry Barnhart
Can the New Way of Working Last for Agile Biopharma?
While this system had results, would it work in the long term? Barnhart shared some pain points he heard after talking to various teams who were involved with creating the vaccine.
- 80-hour work weeks are not sustainable
- Costs will be unsustainable if you take on risks for all products all at once
- Pharma organizations are designed for waiting, with “time slicing” built in to allow other parts to catch up and align
One way to get around these serious concerns is to rethink the structure of the organization in general. Barnhart shared an example of a project he worked on with a printing company, stating that by implementing Agile Biopharma and Lean approaches, the team was able to do more than three years’ worth of work in 60 days. The team had a hard time with that level of production, but no one on the management staff considered spreading out that work over 120 days, even though they still would have accomplished “more than they had done in the past decade.” According to Barnhart, if the organization adapts to Agile and Lean practices, then working hours should be more sustainable and would even generate newfound time that could be spent on new products or new ways to get those products to the market.
In terms of unsustainable costs, Barnhart notes that, through Agile and Lean practices, an organization could run two projects to deliver in half the time and two to deliver at the normal time. Even if two of those projects fail, Barnhart said, “The upside is, you’ve just delivered one of those projects to the market two years earlier. And if you think about what we try to do in pharma, that’s somewhere between 200 million and 6 or 8 billion in sales. It will cover the costs and much, much, much more.”
Speaking of the “much, much, much more,” implementing Agile Biopharma and Lean practices can give patients a new lease on life. Barnhart shared an example of a project he did not work on at Pfizer in which Pfizer got the entirety of the research to go a year faster before FIH, or the first in-human study, meaning the drug launched a year ahead of time. Barnhart said, “We saved or extended the lives of 10 or 15,000 people as a result of that. Tell me there is a scientist in the world that doesn’t want to do this.”
We saved or extended the lives of 10 or 15,000 people as a result of that. Tell me there is a scientist in the world that doesn’t want to do this.
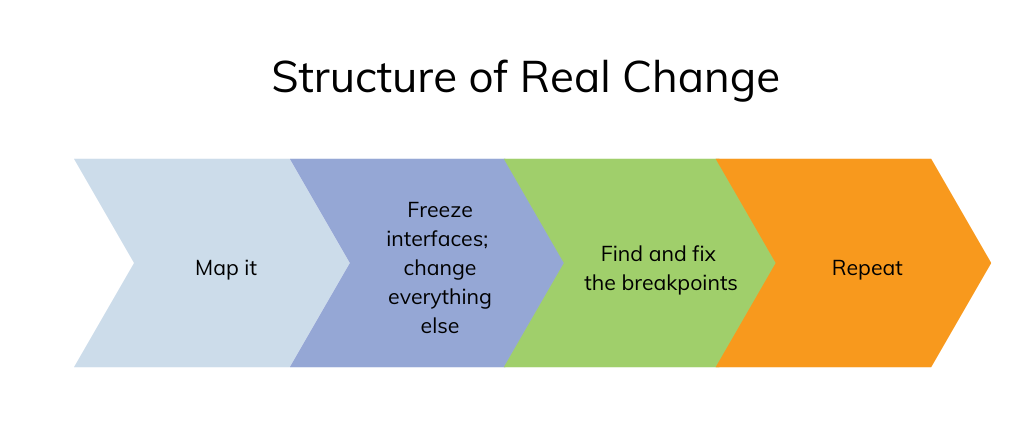
The last concern is what happens when waiting is removed from the equation. At Pfizer, Barnhart worked on a project in which they mapped a program from end to end and collapsed that into a “pure flow, from start to finish, no wait states.” Whenever a team became stressed enough and asked for help, Barnhart’s team would help them line out their processes so they could go at the pace the program needed, which helped all the other programs in the organization gain speed. Ultimately, Barnhart’s team found that a particular process worked: “So what we would do is one value stream from idea to a particular point, collapse that. fix the supporting things that we saw that were breaking, and then repeat.” By the end of three years, Barnhart’s team was able to accelerate the entire Pfizer portfolio by one year.
What Next for Agile Biopharma?
Barnhart noted that, beyond Warp Speed, much work remains to be done, as there are still 2 million people dying of the top 10 causes of death in the United States, minus COVID-19 and accidents.* If you want to help bring Warp Speed to everyone, Barnhart offered two tips:
- Call out the waiting in your own place
- Start connecting with peers who want to solve these problems, including the following:
- Connecting with Barnhart on LinkedIn.
- Connecting with Eliassen Group’s Jen Mariani on LinkedIn so you can participate in our monthly Lean Coffee sessions.
- Joining the Lean Product And Process Development Exchange (LPPDE) group chat on LinkedIn. LPPDE is a nonprofit that works on the science of improving innovation across industries.
Agile Biopharma Takeaways
Before you go, keep the following in mind:
- The entire pharmaceutical industry has tried to bring products to market faster, but only Operation Warp Speed has been effective.
- Although Operation Warp Speed was successful, some teams are concerned that a complete change to Agile methods may be unsustainable.
- These concerns about changing to Agile methods can be mitigated if organizations restructure how long a project runs and how those projects are scheduled in relation to each other.
- Organizations should also consider mapping programs from end to end without wait states to see where they break and then fix those breakpoints.
- If you want to see change in your organization, start joining forces with others in the industry to call out waiting and bring Warp Speed to everyone.
Conclusion
Learn more about our Agile Consulting approach and our Agile & Biopharma experience, and if you are interested in being part of the Agile & Biopharma conversation, join one of our future Lean Coffees or Lunch and Learns.
Sources
“Leading Causes of Death.” Centers for Disease Control and Prevention. Accessed 17 May 2022. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm